Pharmaceutical Injectable Products
ADRIMED Injection


Composition
Each 1 ml contains:
Noradrenaline Bitartrate IP: 2 mg (equivalent to 1 mg Noradrenaline)
Water for Injection IP: 1 ml
Dilute to 250 times the volume with Sodium Chloride and Dextrose Injection or Dextrose Injection (5% w/v) to prepare Noradrenaline Bitartrate Injection. Use immediately after preparation.
Dosage
As directed by the physician.
ADRIMED Injection contains an adrenergic drug used to treat severe hypotension, shock, and cardiovascular emergencies. This injectable solution helps regulate blood pressure by stimulating the heart and blood vessels, ensuring improved blood flow and heart function. ADRIMED is particularly effective in critical care settings, where rapid intervention is necessary to stabilize patients with life-threatening conditions. It works by enhancing heart rate and improving circulation, helping to prevent organ damage due to poor blood flow. The dosage and administration should always be determined by a physician, based on the patient’s specific condition and response to treatment.
ANTILANT-LMWH Injection
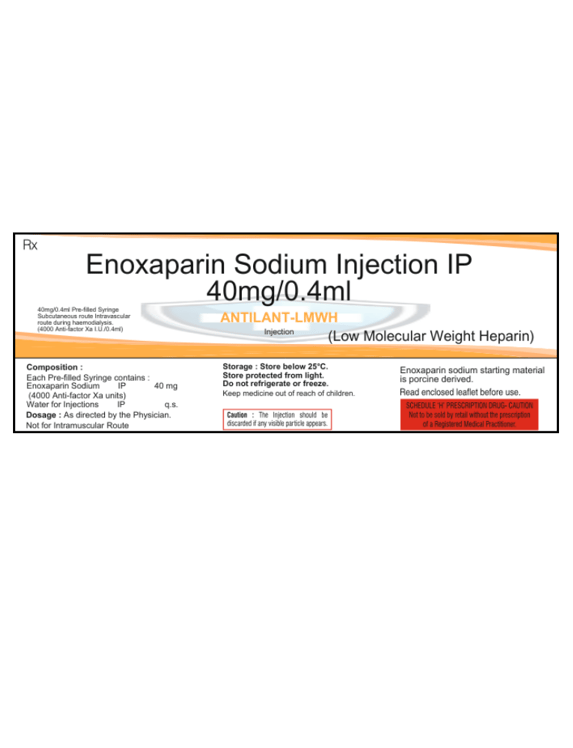
Composition
Each pre-filled syringe contains:
Enoxaparin Sodium IP: 40mg (4000 Anti-factor Xa units)
Water for Injection
Dosage:
As directed by the physician.
Note:
Not for intramuscular use. Please follow the prescribed route of administration.
ANTILANT-LMWH Injection contains Enoxaparin Sodium, a low molecular weight heparin, used to prevent and treat deep vein thrombosis (DVT), pulmonary embolism (PE), and other clotting disorders. It works by inhibiting clot formation through anti-factor Xa activity. This pre-filled syringe ensures precise dosage, with 40mg (4000 Anti-factor Xa units) per 0.4ml of solution. Administered as directed by a physician, it is essential for effective anticoagulation therapy. The injection is not intended for intramuscular use and should be administered according to the prescribed route. Always follow medical guidance for safe and effective use.
ANTILANT-UFH Injection
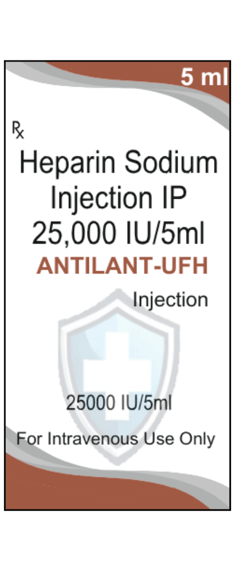

Composition
Each 5 ml contains:
Heparin Sodium IP: 25,000 IU
Water for Injections IP
Dosage:
As directed by the physician.
For intravenous use only.
Caution:
Do not use if foreign particles are present in the vial after dissolving. Discard any unused portion. This vial is for single-dose use only.
Storage:
Store below 30°C in a cool, dry, and dark place.
Keep out of reach of children.
Note:
FLIP OFF SEAL PROVIDED FOR TAMPER EVIDENCE.
ANTILANT-UFH Injection contains Heparin Sodium, a potent anticoagulant used to prevent and treat blood clot formation in various medical conditions. Each 5 ml vial contains 25,000 IU of Heparin Sodium, providing effective prevention of thromboembolic events when administered intravenously, as directed by a physician. It is crucial to follow medical guidance regarding the correct dosage and administration route. The injection is intended for single use only and should be discarded after administration. If any foreign particles are observed, do not use the solution. Store the vial below 30°C in a cool, dry, and dark place.
CLAVTRACK Injection


Composition
Each vial contains:
Amoxycillin Sodium (Sterile) IP: Equivalent to Anhydrous Amoxycillin
Potassium Clavulanate (Sterile) IP: 1.0 gm (Equivalent to 200 mg Clavulanic Acid)
Dosage:
As directed by the physician. For intravenous use only.
Direction for Use:
Reconstitute the vial’s contents with the provided solvent. The reconstituted solution should be used immediately after preparation and must not be frozen.
Storage:
Store in a cool, dry place, protected from light.
Caution:
Keep out of reach of children. Not for retail sale without a prescription from a registered medical practitioner.
CLAVTRACK Injection combines Amoxycillin and Potassium Clavulanate, providing broad-spectrum antibiotic coverage. It is effective in treating infections caused by bacteria resistant to penicillin alone. Each vial contains 1.0 gm of Potassium Clavulanate (equivalent to 200 mg of Clavulanic Acid) and Amoxycillin Sodium, which ensures enhanced efficacy against resistant bacteria. The injection is intended for intravenous use only and should be reconstituted with the provided solvent, to be used immediately after preparation. The solution must not be frozen. Store the product in a cool, dry place, away from light. Always use under physician’s guidance.
CITILOCK Injection
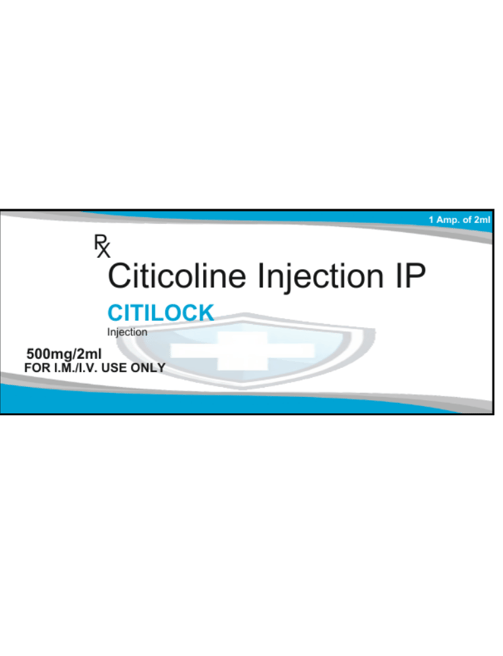
Composition
Each 2 ml ampoule contains:
Citicoline Sodium IP: 500 mg
Methyl Paraben: 0.18% w/v (as preservative)
Propyl Paraben: 0.02% v (as preservative)
Water for Injections I.P.
Dosage:
As directed by the neurologist. For intramuscular or intravenous use only.
Storage:
Store in a cool, dry, and dark place. Keep out of reach of children.
Caution:
Do not use the solution if foreign particles are visible in the ampoule.
CITILOCK Injection contains 500 mg of Citicoline Sodium per 2 ml, designed for intravenous or intramuscular administration. It is used to improve cognitive function and support brain health in conditions such as stroke and neurodegenerative diseases. The injection also contains preservatives (Methyl Paraben and Propyl Paraben) to maintain the solution’s stability. For best results, CITILOCK should be administered as directed by a neurologist. The solution must be free from visible particles before use. Store in a cool, dry, and dark place, away from children. Always consult a healthcare professional before use. Manufactured by Protech Telelinks.
DOBUTOR Injection


Composition
Each 1 ml contains:
Dobutamine Hydrochloride: 50 mg
Water for Injections: q.s. (quantity sufficient)
DOBUTOR Injection should be diluted to the appropriate strength with a suitable parenteral vehicle prior to administration.
Dosage:
As directed by the physician.
Storage:
Store in a cool, dry place, below 25°C. Keep out of reach of children.
Caution:
Do not use the solution if foreign particles are visible in the ampoule.
DOBUTOR Injection contains 50 mg of Dobutamine Hydrochloride per 1 ml, intended for intravenous administration. It is used primarily for short-term treatment of heart failure and to improve cardiac output in conditions such as shock. The injection must be diluted to the appropriate strength with a suitable parenteral vehicle before use. It is essential that the solution is free of foreign particles before administration. Store DOBUTOR in a cool, dry place, below 25°C, and keep out of reach of children. Always follow the dosage instructions provided by the physician. Manufactured by Protech Telelinks, WHO-GMP Certified.
DICLOHOPE-AQ Injection


Composition
Each 1 ml contains:
Diclofenac Sodium IP: 75 mg
Benzyl Alcohol (as preservative)
Water for Injections IP: q.s.
Indications:
For intramuscular, intradeltoid, intragluteal, or IV infusion use, as directed by the physician.
Storage:
Store in a cool, dry place, protected from light and moisture. Do not freeze. Keep out of reach of children.
Dosage:
As prescribed by the physician.
DICLOHOPE-AQ Injection contains 75 mg of Diclofenac Sodium per 1 ml, along with Benzyl Alcohol as a preservative and Water for Injections. It is designed for intramuscular, intradeltoid, intragluteal, or intravenous infusion use, as directed by a healthcare professional. This medication is used for pain relief and inflammation control. Store in a cool, dry place, away from light and moisture, and do not freeze. Keep out of reach of children. Always follow the physician's prescribed dosage for optimal results. Manufactured by Protech Telelinks, a WHO-GMP certified company.
DOXITRACK Injection


Composition
Each vial contains:
Doxycycline Hyclate (Sterile) IP, equivalent to Doxycycline: 100 mg
Indications:
For intravenous (IV) infusion use only, as directed by the physician.
Storage:
Store below 25°C, protected from light. Keep out of reach of children. If any foreign particles are visible after reconstitution, do not use the solution.
Dosage:
As prescribed by the physician.
DOXITRACK Injection contains Doxycycline Hyclate (Sterile) IP, equivalent to 100 mg of Doxycycline. It is used for the treatment of bacterial infections and is administered via intravenous (IV) infusion as directed by a physician. The vial should be stored below 25°C, protected from light, and kept out of reach of children. If any foreign particles are visible after reconstitution, the solution should not be used. Always follow the prescribed dosage for safe and effective treatment.
LEVETRACK Injection


Composition
Each ml contains:
Levetiracetam
Water for Injections IP
Indications:
For intravenous (IV) infusion use only, as directed by the neurologist.
Storage:
Store in a cool, dry place, protected from light, below 25°C. To be diluted prior to IV infusion. Keep out of reach of children.
Caution:
If any foreign particles are visible in the vial after dissolving the contents, do not use the solution.
Dosage:
As prescribed by the neurologist.
LEVETRACK Injection is used for intravenous (IV) infusion, as prescribed by the neurologist. Each vial contains Levetiracetam and Water for Injections IP, formulated for safe and effective administration. It is essential to dilute the solution before use. Store the injection in a cool, dry place, protected from light, and below 25°C. Keep it out of reach of children. If any foreign particles appear after dissolving, do not use the solution. Always follow the dosage instructions provided by your physician for optimal results. Manufactured by Protech Telelinks, a WHO-GMP certified company.
MAJICORT Injection


Composition
Each vial contains Hydrocortisone Sodium Succinate IP, equivalent to Hydrocortisone (Sterile Lyophilized Powder).
Dosage:
As directed by the physician.
Directions for Use:
Dissolve the contents of the vial in 2 ml of Sterile Water for Injections I.P. for intramuscular (IM) use or use a suitable diluent for intravenous (IV) infusion. The reconstituted solution should be used immediately after preparation.
Storage:
Store in a cool, dry place, protected from moisture, in a single-dose container at a temperature not exceeding 30°C.
Caution:
If any foreign particles are visible in the vial after dissolving the contents, do not use the solution.
This pack also includes one FFS Ampoule of Sterile Water for Injections I.P. 5 ml.
Keep out of reach of children.
MAJICORT Injection contains Hydrocortisone Sodium Succinate, equivalent to Hydrocortisone (Sterile Lyophilized Powder). It is used as prescribed by your physician for intramuscular (IM) or intravenous (IV) administration. To use, dissolve the powder in 2 ml of Sterile Water for Injections I.P. for IM use or an appropriate diluent for IV infusion. The solution should be used immediately after preparation. Store in a cool, dry place, away from moisture, and at a temperature not exceeding 30°C. Keep out of reach of children. Consult your physician for dosage and further instructions.
MEDVERINE Injection
Composition
Each 1 ml contains:
Drotaverine Hydrochloride IP, equivalent to Drotaverine: 20 mg
Water for Injections (q.s.)
Indications:
For intramuscular (IM) or intravenous (IV) use, as prescribed by the physician.
Dosage:
As directed by your physician.
Storage:
Store in a cool, dark place, protected from light. Keep out of reach of children. Do not use if foreign particles are visible in the solution.
MEDVERINE Injection is used for intramuscular (IM) or intravenous (IV) administration, as directed by the physician. Each 1 ml of the injection contains 20 mg of Drotaverine Hydrochloride, equivalent to Drotaverine, and water for injections. This medication is primarily used to relieve smooth muscle spasms. It should be stored in a cool, dark place, protected from light, and kept out of the reach of children. Before use, ensure no foreign particles are present in the solution. Always follow the dosage prescribed by your healthcare provider. MEDVERINE Injection is manufactured by Protech Telelinks, a WHO-GMP certified company.
MEROTRACK Injection


Composition
Each vial contains:
Meropenem (Sterile), equivalent to Anhydrous Meropenem 1000 mg
Sodium Carbonate (Sterile), equivalent to Sodium
A sterile mixture of Meropenem IP & Sodium Carbonate IP
Indications:
For intravenous (IV) use only, as prescribed by the physician.
Dosage:
As directed by your physician.
Storage:
Store protected from moisture at a temperature not exceeding 30°C. Keep out of reach of children. Do not use if foreign particles are visible in the solution.
Direction for use:
Reconstitute with 20 ml of Sterile Water for Injections IP for IV use. Any unused reconstituted portion must be discarded.
MEROTRACK Injection is used for intravenous (IV) infusion, as prescribed by the physician. Each vial contains Meropenem (equivalent to Anhydrous Meropenem 1000 mg) and Sodium Carbonate, formulated for safe and effective use. The powder should be reconstituted with 20 ml of Sterile Water for Injections IP before administration. Store the injection in a cool, dry place, protected from light, and below 30°C. Keep it out of reach of children. If any foreign particles are present after dissolving, do not use the solution. Always follow the dosage instructions provided by your physician for best results. Manufactured by Protech Telelinks, a WHO-GMP certified company.
NITROCHECK Injection


Composition
Each ml contains:
Nitroglycerin IP: 5 mg
Water for Injections IP: q.s.
Indications: For intravenous (IV) infusion only. Must be diluted before use, as prescribed by the physician.
Dosage: As directed by your physician.
Storage: Store in a cool, dry place, below 25°C. Protect from light and moisture. Do not allow to freeze. Keep out of reach of children.
Caution: Do not use if discolored or if a precipitate is present. Avoid contact with alkalies. Any unused solution must be discarded.
SCHEDULE H PRESCRIPTION DRUG-CAUTION: Not to be sold by retail without the prescription of a Registered Medical Practitioner.
NITROCHECK Injection is used for intravenous (IV) infusion, as prescribed by the physician. Each ml contains Nitroglycerin IP (5 mg) and Water for Injections IP, formulated for effective use. The solution must be diluted before use. Store the injection in a cool, dry place, below 25°C, protected from light and moisture. Do not allow the solution to freeze. Keep it out of reach of children. Do not use if the solution is discolored or contains precipitate. Always follow the dosage instructions provided by your physician for optimal results.
PROFCID Injection


Composition
Each vial contains:
Pantoprazole Sodium I.P., equivalent to Pantoprazole (Sterile Lyophilized Powder)
This pack also contains one FFS ampoule of Sterile Sodium Chloride Injection I.P. 10 ml
Indications: For intravenous (IV) infusion use only, as prescribed by the physician.
Dosage: As directed by your physician.
Storage: Store below 25°C, protect from sunlight. Do not freeze. Keep out of reach of children.
Caution: Do not use if discolored or if a precipitate is present. Discard any unused solution.
SCHEDULE H PRESCRIPTION DRUG-CAUTION: Not to be sold by retail without the prescription of a Registered Medical Practitioner. Keep out of reach of children.
PROFCID Injection is used for intravenous (IV) infusion, as prescribed by the physician. Each vial contains Pantoprazole Sodium I.P., equivalent to Pantoprazole (Sterile Lyophilized Powder). The solution must be reconstituted with 10 ml of Sterile Sodium Chloride Injection I.P. provided in the pack. Store the injection in a cool, dry place, below 25°C, protected from sunlight. Do not freeze the solution. Keep it out of reach of children. Do not use if the solution is discolored or contains precipitate. Always follow the dosage instructions provided by your physician for effective results.
TEICOGOLD Injection

Composition
Each vial contains:
Teicoplanin IP 400 mg
(Sterile Lyophilized Powder)
Indications: For intravenous (IV) infusion use only, as prescribed by the physician.
Dosage: As directed by your physician.
Storage: Store in a dry place below 25°C. Protect from light. Keep medicine out of reach of children.
Direction for Use: Dissolve the contents of the vial in 3 ml of Sterile Water for Injections IP.
Caution: If any foreign particle is visible in the vial after dissolving the content, please do not use the solution.
SCHEDULE H PRESCRIPTION DRUG-CAUTION: Not to be sold by retail without the prescription of a Registered Medical Practitioner. Keep out of reach of children.
TEICOGOLD Injection is used for intravenous (IV) infusion, as prescribed by the physician. Each vial contains Teicoplanin IP 400 mg (Sterile Lyophilized Powder). The solution must be reconstituted with 3 ml of Sterile Water for Injections IP. Store the injection in a cool, dry place, below 25°C, protected from light. Do not freeze the solution. Keep it out of reach of children. Do not use if any foreign particles are visible after dissolving the content. Always follow the dosage instructions provided by your physician for optimal results.
TRANGOLD Injection


Composition
Each ml contains:
Tranexamic Acid IP: 100 mg
Water for Injection IP: q.s.
Indications: For intravenous (IV) use only, as prescribed by the physician.
Dosage: As directed by your physician.
Storage: Store in a cool, dry, and dark place, below 25ºC. Protect from light.
Caution: Do not use if the contents are not clear or show particulate matter. Discard ampoule if the solution is colored.
SCHEDULE ‘H’ PRESCRIPTION DRUG-CAUTION: Not to be sold by retail without the prescription of a Registered Medical Practitioner. Keep medicine out of reach of children.
TRANGOLD Injection is used for intravenous (IV) infusion, as prescribed by the physician. Each vial contains Tranexamic Acid IP 500 mg (Sterile Solution). The solution must be used as directed by your physician. Store the injection in a cool, dry, and dark place, below 25ºC, protected from light. Do not freeze the solution. Keep it out of reach of children. Do not use if the solution is discolored or contains particulate matter. Always follow the dosage instructions provided by your physician for optimal results.
VOMIDOC Injection
Composition
Each 2ml contains:
Ondansetron Hydrochloride IP, equivalent to Ondansetron: 4 mg
Methylparaben (As Preservative) IP: 0.18% w/v
Propylparaben (As Preservative) IP: 0.02% w/v
Water for Injections IP: q.s.
Indications: For intramuscular (IM) or intravenous (IV) use only, as prescribed by the physician.
Dosage: As directed by your physician.
Storage: Store in a cool place, protected from heat and light.
Caution: Keep medicine out of reach of children. Do not use if the solution is discolored or contains visible particulate matter. Always follow the dosage instructions provided by your physician.
VOMIDOC Injection is used for intravenous (IV) or intramuscular (IM) injection, as prescribed by the physician. Each 2 ml contains Ondansetron Hydrochloride IP, equivalent to Ondansetron 4 mg, along with preservatives (Methylparaben 0.18% w/v and Propylparaben 0.02% w/v). Store the injection in a cool place, protected from heat and light. Do not freeze the solution. Keep it out of reach of children. Do not use if the solution is discolored or contains visible particulate matter. Always follow the dosage instructions provided by your physician for optimal results.
GOLOXINE Injection

Composition
Each vial contains:
Ceftriaxone I.P.: 1g (Sterile Powder for Injection)
Suitable excipients for reconstitution.
Dosage: As directed by your physician.
Storage: Store in a cool, dry place, below 25°C. Protect from light and moisture. Do not freeze the solution.
Caution: Keep medicine out of reach of children. Do not use if the solution is discolored or contains visible particulate matter. Always follow the dosage instructions provided by your physician for best results.
GOLOXINE Injection contains Ceftriaxone I.P., an antibiotic used to treat various bacterial infections. Each vial contains 1g of Ceftriaxone in sterile powder form. It is reconstituted with the prescribed diluent for intravenous (IV) or intramuscular (IM) use. Ceftriaxone works by inhibiting the growth of bacteria, helping the body fight infections effectively. The dosage and duration of treatment depend on the infection type and your physician's recommendation. Store the vial in a cool, dry place, away from light. Follow your doctor’s instructions carefully to ensure optimal results. Keep out of reach of children.
CEFUROGOLD Injection


Composition
Each vial contains:
Cefuroxime I.P.: 1.5 gm
Water for Injection I.P.: q.s.
Indications: For intravenous (IV) or intramuscular (IM) use only, as prescribed by the physician.
Dosage: As directed by your physician.
Storage: Store in a cool, dry, and dark place, below 25ºC. Protect from light.
Caution: Do not use if the contents are not clear or show particulate matter. Discard ampoule if the solution is colored.
SCHEDULE ‘H’ PRESCRIPTION DRUG-CAUTION: Not to be sold by retail without the prescription of a Registered Medical Practitioner. Keep medicine out of reach of children.
CEFUROGOLD Injection is a sterile solution containing 1.5 gm of Cefuroxime I.P. and is indicated for intravenous (IV) or intramuscular (IM) use, as prescribed by your doctor. It is used for treating various bacterial infections. The solution must be prepared as directed and administered under medical supervision. Store the injection in a cool, dry, and dark place, ensuring it stays below 25ºC and is protected from light. The solution should not be used if it appears discolored or contains particles. Always follow your physician's instructions for proper dosage and usage. Keep out of reach of children.
MIKALOCK Injection


Composition
Each vial contains:
Amikacin Sulphate I.P.: 500 mg
Water for Injection I.P.: q.s.
Indications: MIKALOCK Injection is used for intravenous (IV) or intramuscular (IM) administration, as prescribed by the physician. It is used to treat various bacterial infections.
Dosage: As directed by your physician.
Storage: Store in a cool, dry place, below 25ºC, protected from light.
Caution: Do not use if the solution is discolored or contains particulate matter. Keep the medicine out of reach of children. Always follow the dosage instructions provided by your healthcare provider.
SCHEDULE 'H' PRESCRIPTION DRUG-CAUTION: Not to be sold by retail without the prescription of a Registered Medical Practitioner.
MIKALOCK Injection is used for intravenous (IV) or intramuscular (IM) administration to treat bacterial infections, as prescribed by your healthcare provider. Each vial contains 500 mg of Amikacin Sulphate I.P., formulated with water for injection. The dosage and duration of treatment should follow the specific instructions provided by your physician. For optimal results, it is essential to use the injection exactly as directed. Store the injection in a cool, dry place below 25ºC and protect it from light. Do not use the solution if it is discolored or contains visible particles. Keep out of reach of children.
PACILOCK-IV Infusion


Composition
Each 100 ml contains:
Paracetamol IP: 1000 mg
Water for Injections IP: q.s.
Indications: For intravenous (IV) use only, as prescribed by your physician.
Dosage: As directed by your physician.
Storage: Store in a cool, dry place below 25ºC. Protect from light.
Caution: Keep medicine out of reach of children. Do not use if the solution is discolored or contains particulate matter. Always follow the dosage instructions provided by your physician for optimal results.
PACILOCK-IV Infusion is used for intravenous administration, providing effective pain and fever management. Each 100 ml contains 1000 mg of Paracetamol IP, formulated in a sterile solution of Water for Injections IP. This infusion is intended for use as prescribed by your physician to ensure precise dosage and optimal treatment. Store the infusion in a cool, dry place below 25ºC and protect it from light. The solution should not be used if discolored or contains visible particulate matter. Always adhere to the prescribed dosage and safety guidelines for the best therapeutic outcome. Keep out of reach of children.
Medi Hero Biotech - Your Ideal Trusted Medical Partner
At MEDI HERO BIOTECH LLP, we are dedicated to delivering superior pharmaceutical solutions that healthcare professionals can rely on. Our injectables are manufactured under stringent quality standards, ensuring safety, stability, and efficacy in every dose.
quik link
208/1,N.N Road, Kol-28
+91 82404 37563
© 2025. All rights reserved.
get in touch
